Figure A and B
Sensogram showing binding of mouse wild-type and Fc SilentTM anti-F4/80 IgG2a monoclonal antibodies (Ab00106-2.0 and Ab00106-2.3 respectively) to immobilized mouse low and high affinity Fcg Receptors (CD16, CD16-2, CD32, CD64). BIAcore SPR binding analysis shows that the Fc SilentTM antibody (Ab00106-2.3) has lost its capacity to interact with the mouse Fc Receptors (Figure B) (data provided by Dr Stephen Beers, University of Southampton).
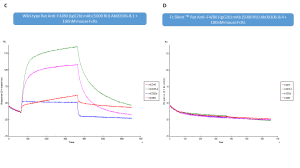 Figure C and D
Figure C and D
Sensogram showing binding of rat wild-type and Fc SilentTM anti-F4/80 monoclonal antibodies (Ab00106-8.1 and Ab00106-8.4 respectively) to immobilized mouse low and high affinity Fcg Receptors (CD16, CD16-2, CD32, CD64). BIAcore SPR binding analysis shows that the Fc SilentTM antibody (Ab00106-8.4) has lost its capacity to interact with the mouse Fc Receptors (Figure D) (data provided by Dr Stephen Beers, University of Southampton).
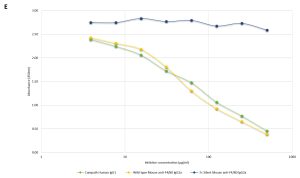 Figure E Direct Competitive Inhibition ELISA
Figure E Direct Competitive Inhibition ELISA
2µg/ml/well of human recombinant FcgR1 receptor (rCD64) was absorbed onto a 96-well plate. 40µg/ml biotinylated Alemtuzumab (Campath) was premixed with un-labelled serially diluted murine wild-type or Fc SilentTM anti-F4/80 [CI:A3-1] IgG2a monoclonal antibodies (Ab00106-2.0 or Ab00106-2.3 respectively) or Alemtuzumab (Campath; positive control). The data clearly shows that the Fc SilentTM antibody, Ab00106-2.3, does not bind to the human Fc receptor.

 United Kingdom (UK)
United Kingdom (UK)