With a coronavirus vaccine still in development and the worldwide economy reeling from the pandemic, business leaders around the world are being tested on how exactly to respond to this new normal. COVID-19 has radically changed the delivery of products and services in every sector, and at the same time exposed failings in the assumptions that guided global supply chain dogma.
Companies with adaptable processes have been less affected by this shock than others. This concept is often called “operational resiliency” – the idea that an organization can quickly and flexibly adapt in response to external disruptions beyond its control. Companies with strong operational resilience are better positioned to handle changing conditions due to the coronavirus pandemic as well as any future challenges.
For scientific and diagnostic companies, having a secure supply chain of high-quality reagents is critical for weathering unforeseen circumstances. Recombinant antibody technology has historically been reserved for therapeutic companies in their development of novel biologics; however, the technology at its core mitigates manufacturing risk by “digitizing” antibody assets and guaranteeing long-term reproducible production. It also increases the ability to manipulate assets for any specific bio-physical requirements that might arise.
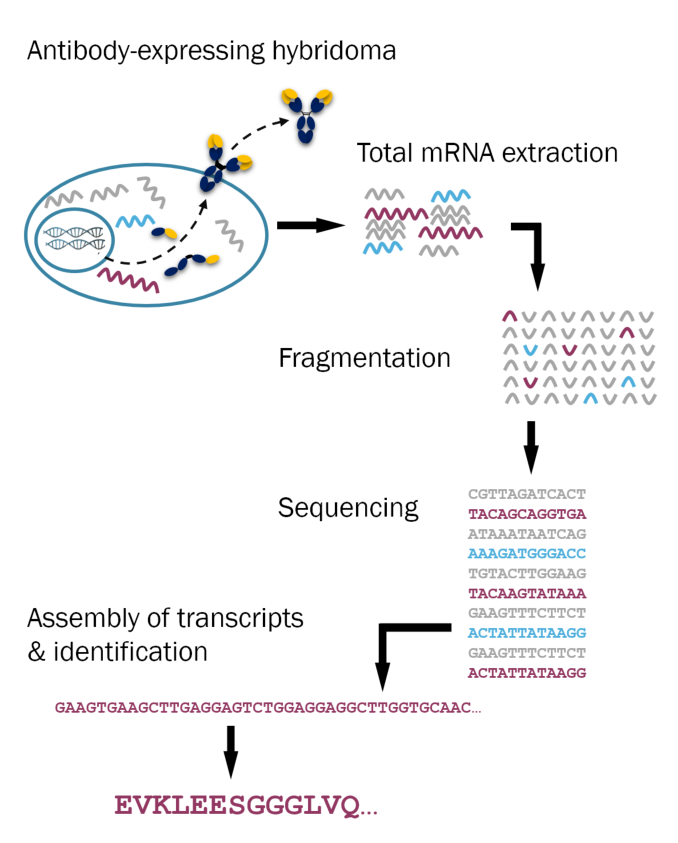
Sequencing enables antibodies to be manufactured recombinantly for the entire lifespan of an assay. The above describes our hybridoma sequencing method; antibody protein and polyclonal sequencing are also available.
Implementing recombinant antibody technology therefore strengthens operational resilience in three ways:
- Decreased reliance on a physical asset. By sequencing your antibody assets, the antibodies are no longer physically or geographically constrained to a centralized facility. Digital antibody sequences can be easily accessed by multiple stakeholders around the world, allowing companies to exploit the flexibility of regional supply chains. If your main manufacturing facility must shut down, other facilities can use the antibody sequences to continue production. The same exact antibody can therefore be manufactured anywhere in the world, even if the original antibody is lost, mutated or contaminated.
- Flexibility to make or buy. Make versus buy is a question faced by every manager from large international companies to the individual academic lab. In today’s new normal of social distancing, the limited number of people allowed in a lab can hamper productivity; resources should therefore be focused on core competencies to maximize output. Most labs have in-house expression, but is that a core competency of your organization? Could limited resources be allocated elsewhere and antibody expression outsourced? With recombinant antibody technology, outsourcing is simple: again, all antibody manufacturing will start from the same digital sequence for ensured batch-to-batch reproducibility.
- Agile response to reagent requirements. One of the biggest benefits to recombinant antibody technology is the ability to quickly change antibody formats, isotypes, subtypes and species with minimal effect on binding specificity. This opens up possibilities never before considered when working with hybridoma technology. As a recent example, diagnostic developers could quickly create anti-SARS-CoV-2 antibodies in different species, isotypes and subtypes for use in COVID-19 assays. The downside is that we can fall into a state of choice overload; however, that is where experts can add value by assisting with selecting strategies that have the greatest probability of success.
Even during this titanic shift in the business landscape, we have seen companies quickly adapt and implement new strategies that maintain productivity while preserving cash flow. This flexible adaption comes from having processes that are resilient to shocks to normal operation. As we emerge into a post-COVID landscape, it is an opportunity to re-visit business practices to maximize operational resilience.
Recombinant antibody technology can be a solution for maximizing efficiency and productivity, as well as strengthening an antibody supply chain by shifting assets from a centralized facility to the cloud. This is one of the reasons Absolute Antibody has endeavored since our founding in 2012 to make recombinant antibody technology accessible to all.
To learn more about how you can easily and efficiently convert your hybridomas to recombinant antibodies, book a time with Absolute Antibody.
Latest News
Upcoming Events
Please join us at the following conferences and events. Stop by our booth, or get in touch to arrange a meeting.
See All Dates
 United Kingdom (UK)
United Kingdom (UK)