Project Summary: Dr. Mariano Viapiano of the State University of New York had developed an antibody that successfully treated glioblastoma in mouse models. Absolute Antibody provided recombinant production, chimerization and finally humanization services to develop optimized humanized antibodies with the robust manufacturability required for clinical use.
A New Glioblastoma Target
Dr. Mariano Viapiano, Associate Professor of Neuroscience at the State University of New York (Upstate Medical Center, Syracuse, NY), studies the neural extracellular matrix (ECM) and its impact on malignant brain cancers. By targeting signaling molecules localized to the ECM, his lab had developed a mouse monoclonal antibody with the potential to treat glioblastoma. Peer-reviewed research showed the antibody successfully reduced tumor growth and increased survival rates in mice with glioblastoma.
Despite this promising functionality in mouse models, Dr. Viapiano knew the antibody would need reformatting to move it toward clinical use. He sequenced the hybridoma to prepare for recombinant expression and enable antibody optimization and scaled-up production.
Recombinant Production and Chimerization
Dr. Viapiano researched companies offering recombinant antibody production, and he selected Absolute  Antibody for our competitive pricing and detailed upfront explanations of what the process would entail. We then used our HEXpress™ transient expression system to manufacture the recombinant antibodies, which displayed the same glioblastoma-targeting properties as the original hybridoma-derived version. However, the antibody still struggled with solubility and other issues that would impede its therapeutic use.
Antibody for our competitive pricing and detailed upfront explanations of what the process would entail. We then used our HEXpress™ transient expression system to manufacture the recombinant antibodies, which displayed the same glioblastoma-targeting properties as the original hybridoma-derived version. However, the antibody still struggled with solubility and other issues that would impede its therapeutic use.
As a result, Dr. Viapiano began consulting with the Absolute Antibody team on how to best improve the antibody’s performance. Together, the decision was reached to chimerize the antibody.
“Absolute Antibody doesn’t do services in isolation,” Dr. Viapiano said. “It’s almost like collaborating with another academic lab, with the advantage that you get a product at the end. The turnaround time is very quick, the product has outstanding qualities, and we were able to interact closely throughout the entire antibody optimization process.”
Moving to Antibody Humanization
Dr. Viapiano began experiments with the chimerized antibody, but it still demonstrated manufacturability properties unsuitable for large-scale therapeutic use, in particular precipitation and weak expression. Attempts to modify the antibody backbone did not effectively address the problem, and Absolute Antibody recommended antibody humanization as the next step.
“I didn’t go into this with humanization in mind,” Dr. Viapiano said. “I thought it’d be out of our reach as a small academic lab.” However, because Absolute Antibody had already accumulated a lot of knowledge about the antibody from our ongoing consultations, we were able to offer a reasonable price the lab could afford.
We therefore began the antibody humanization process using our novel Prometheus™ platform, which guarantees to produce a humanized variant with comparable activity to the parent antibody. This royalty-free humanization service focuses on enhancing antibody manufacturability, leading to increased expression, lower aggregation, and long-term stability and solubility.
Improving Antibody Manufacturability
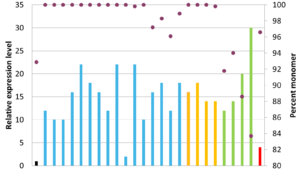
Expression yields relative to the chimeric antibody shown as bars and monomer content shown as purple dots. Black bar is chimeric antibody, blue bars have favourable VH and VL, yellow bars have favourable VL only and red bar has unfavourable VH and VL.
We used structure-guided CDR grafting onto preferred germline backbones to create 25 different humanized variants. All humanized variants demonstrated specific antigen binding and increased titers compared to the chimeric version. Based on activity, titer and aggregation, the three most promising variants were selected for future study. The three candidates displayed no detectable aggregation, an approximately two-fold increase in activity, and a minimum 16-fold increase in titer compared to the chimeric antibody.
Dr. Viapiano’s lab is currently conducting the final experiments to choose a winning variant. The humanized antibody can now be produced in large amounts, enabling safety tests and eventually clinical trials. “Now that we have an actual humanized antibody, our goal is to advance it straight to the clinic,” he said.

Dr. Viapiano has been partnering with Absolute Antibody for more than three years to optimize this antibody and fulfill its potential for treating glioblastoma. “I would absolutely recommend Absolute Antibody to another researcher,” he said. “I was kept informed of the project at each step and milestone, and the team is very efficient. Whenever they said they’re making X antibody with X features, that’s exactly what we got.”
You can download this case study as a PDF here.
Read more about Dr. Viapiano’s research on his laboratory webpage here.
Latest News
Upcoming Events
Please join us at the following conferences and events. Stop by our booth, or get in touch to arrange a meeting.
See All Dates
 United Kingdom (UK)
United Kingdom (UK)