Written collaboratively by Ian Wilkinson, CSO, and Michael Fiebig, VP Product Portfolio & Innovation
Over the last 10+ years, there has been a dramatic rise in the exploitation of bispecific antibodies with at least 89 currently in clinical development and three that have obtained regulatory approval. In theory, the concept is simple, combine the antigen targeting two monoclonal antibodies into one single molecule to develop an extremely potent drug. However, the practice of actually making a bispecific is much more challenging. Mixing two heavy chains and two light chains together results in random pairing and a highly heterogenous mix of species, most of which are not the correctly formed bispecific. This challenge has resulted in a vast range of ingenious solutions.
Our own Periodic Table of Antibodies contains at least 40 different designs for bispecific antibodies, and even this underestimates the number of designs that have been reported in the literature – we estimate this number to be well above 100.
One of the questions we are asked most frequently is “Which bispecific design is the best?”. Unfortunately, there is no straight forward answer. The optimal design will depend on a range of things, including target antigens, their location (soluble or cell surface), concentration in the solution or density on the cell surface, specific epitopes the antibodies bind to on the respective antigens, desired half-life, mechanism of action (blocking, cell killing by ADCC/CDC, CD3 engagement, etc.), and more. There is no one size fits all bispecific design.
When starting a bispecific project, it is important to understand this and as much as possible tailor your bispecific to your particular application. Although many of the parameters may be unknown, we try to address this design phase with a few simple questions:
- What is your desired half-life – short, medium or long?
- Do you want Fc effector function – for example, ADCC and/or CDC?
- Have you considered the impact of avidity?
The first two questions determine the presence or absence of the Fc domain and any mutations that may need to be incorporated. Although a long half-life is often desirable, this is not always the case. Assuming a long half-life is required, then utilizing the naturally long serum persistence of the Fc domain makes sense over other options such as albumin fusion, albumin binding domains or PEGylation. The presence of the Fc domain will typically lead to engagement with Fc receptors and C1q, but this can be modified through well-known mutations if ADCC/CDC are not required.
The third question can be more challenging to answer. What this is aimed at understanding is how many “arms” of the antibody you want binding to each antigen. Although people often think of a bispecific as the classic Y-shaped IgG, one Fab arm binding antigen A and the other binding antigen B, this does not have to be the case. This kind of design is known as a 1:1 binder but you can also generate 2:1 and 2:2 binders. For some targets more binding arms may be better to increase avidity. However, for some targets this can be detrimental.
This is particularly true for CD3e where it is now widely accepted that you don’t want to over-engage CD3e, as this leads to increased systemic toxicity. This moderate binding can be achieved by just having one binding arm or by using a CD3e binding antibody with only a modest affinity. Therefore, in the literature you will often see researchers working with 1:1 or 2:1 bispecifics for T-cell recruitment.
The answers to these questions can help guide you to a narrower set of designs options, but the landscape is still complex. To truly find the most optimal design, you would test all the options, but this can take more time, effort and money than most people are willing to spend.
Based on many years of experience working with clients in the bispecific space, we have come to the conclusion that having a small panel of recommended designs is helpful. This does not mean to say that we can’t work with other formats, of course we can, and our Periodic Table of Antibodies demonstrates this very clearly. However, for customers taking their first steps into bispecifics, it can be helpful to simplify the complexity down to a narrow range of options.
Therefore, we recommend the three designs shown in the image below as our “go to” formats for bispecifics. All are IP-free, giving freedom to operate, they utilise a single chain Fv for one of the specificities to avoid issues associated with light chain shuffling, and they use a knob-into-hole platform to promote Fc heterodimerization where this is required. As a result, high titres of the desired bispecific can be obtained. All three recommended formats contain an Fc domain since we have found that the vast majority of clients want a long half-life for their drug and tend to incorporate some form of Fc silencing to reduce Fc gamma receptor binding and avoid off-target trispecific binding.
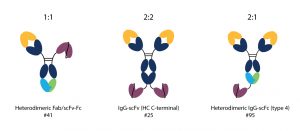
Recommended formats for bispecific antibodies. Numbers represent locations in the Periodic Table of Antibodies.
If you are embarking on a bispecific antibody project and feel a little daunted by the wealth of formats out there, we hope that this gives you a starting point to begin thinking about different approaches. Want to try something different? Contact us to schedule a call with our team to discuss what protein engineering and protein purification solutions Absolute Antibody can offer to get your project off the ground.
Latest News
Upcoming Events
Please join us at the following conferences and events. Stop by our booth, or get in touch to arrange a meeting.
See All Dates
 United Kingdom (UK)
United Kingdom (UK)