Antimicrobial Resistance
According to the World Health Organization, the rise in antimicrobial resistance (AMR) is a growing global threat that is “diminishing the efficacy of common antibiotics against widespread bacterial infections.” It is estimated that 1.27 million global deaths in 2019 can be directly attributed to bacterial AMR (1). Researchers across a number of industries are competing in a microscopic arms race to outmaneuver the rapidly evolving enemy. The good news is that interest in developing non-traditional antibacterials, such as antibodies and bacteriophages, is increasing.
Researchers at the University of Southern California are contributing to the ongoing fight against antimicrobial resistant bacteria. In a recently published paper, “Clinical assays rapidly predict bacterial susceptibility to monoclonal antibody therapy”, the scientists describe how they developed and validated two assays for determining susceptibility, or resistance, of Acinetobacter baumannii to four anti-A. baumannii antibodies.
What is Acinetobacter?
Acinetobacter baumannii is a bacterial pathogen associated with hospital-derived infections. The aerobic, pleomorphic, and non-motile Gram-negative bacillus has been described as “opportunistic” due to its high incidence among hospitalized and immunocompromised individuals, as well as its prevalence in conflict zones (2). The high incidence of antimicrobial resistance found in Acinetobacter is cause for alarm, especially in hospital settings. Carbapenem-resistant Acinetobacter is listed in the CDC’s Antibiotic Resistance Threats in the United States 2019 report (3) and is estimated to have infected 8,500 patients in 2017, resulting in an estimated 700 deaths.
Development and Humanization of New A. baumannii Antibody
Having previously developed three anti-A. baumannii antibodies, the researchers sought to produce a new antibody to expand binding capabilities. Using 30 various strains of A. baumannii, they generated an IgG2a monoclonal antibody, referred to as MAb10. This new antibody showed “binding against 151 of 300 A. baumannii strains from the United States, and 72 of 250 strains from international sources, as assayed via flow cytometry.” Based on the success of the initial tests, the researchers decided to humanize the antibody with Absolute Antibody’s Prometheus™ humanization service.
After the antibody was humanized, flow cytometry testing showed that “the binding and protective efficacy [of the antibody] were maintained.” Further testing against 30 different A. baumannii isolates showed that in every test, except for one, the binding of the humanized antibody was comparable to the original MAb10. The humanized MAb10 and previously developed antibodies were then utilized in two clinical assays developed by the researchers: a high-throughput, single-incubation flow cytometry assay and a latex bead agglutination assay. The researchers believe that these assays may “be useful to help discovery, development, and clinical deployment of MAbs for a variety of microbial pathogens in the future.”
Antibody Humanization
Antibody humanization is a critical step in the development of therapeutic antibodies derived from non-human sources. A technical engineering process, humanization involves transferring critical non-human amino acids onto a human antibody framework. This primarily includes the grafting of amino acids in the complementarity-determining regions (CDRs) but also other framework amino acids critical for the variable heavy:variable light (VH:VL) interface and CDR orientation.
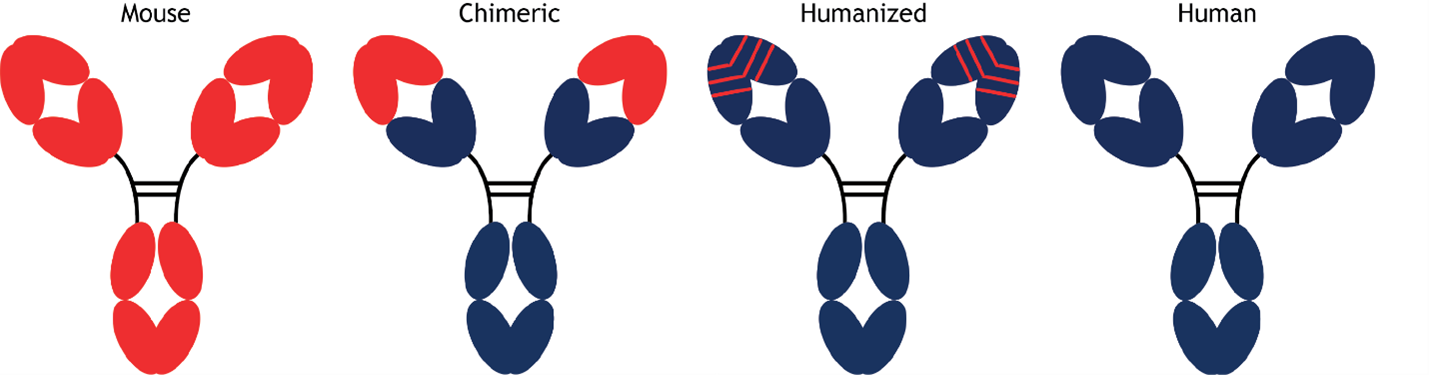
Figure. Progressive humanization of antibodies.
A schematic representation of the advancement from fully mouse antibodies, represented by red domains, to fully human antibodies, represented by blue domains.
Absolute Antibody’s Prometheus™ humanization service was designed with increased antibody manufacturability in mind. The proprietary system, founded on our deep antibody humanization expertise, allows us to offer humanization of murine antibodies, as well as non-murine origin species including rabbit and single-domain camelid nanobodies. We guarantee to produce a panel of humanized variants, and we have completed more than 145 successful humanization projects to date. Our team of expert scientists will work with you to design a variant guaranteed to have comparable activity to the parent antibody.
Learn more about our custom antibody services or contact us to discuss your next antibody project.
References
1. World Health Organization. (2023, November 11). Antimicrobial resistance. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
2. Howard, A., O’Donoghue, M., Feeney, A., & Sleator, R. D. (2012). Acinetobacter baumannii. Virulence, 3(3), 243–250. https://doi.org/10.4161/viru.19700
3. CDC. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019. http://dx.doi.org/10.15620/cdc:82532
Latest News
Upcoming Events
Please join us at the following conferences and events. Stop by our booth, or get in touch to arrange a meeting.
See All Dates
 United Kingdom (UK)
United Kingdom (UK)