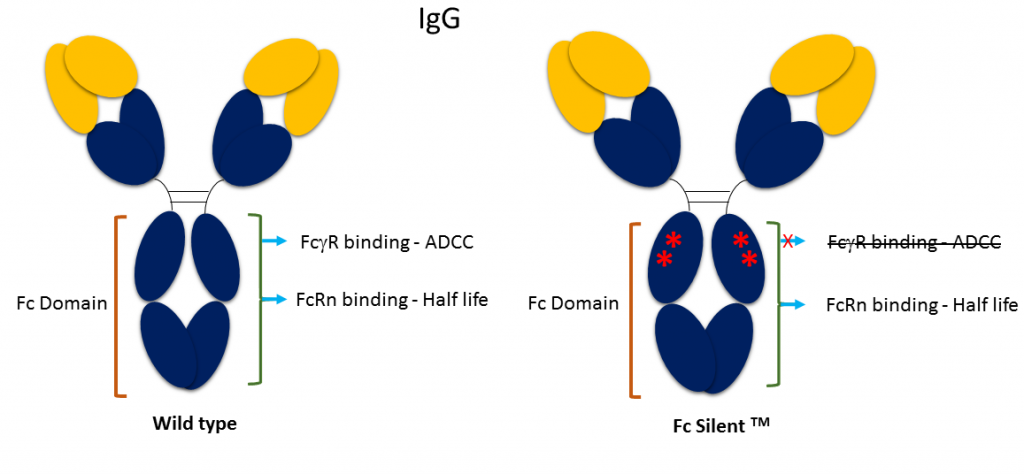
 Fc Silent™ represents a genetically engineered Fc domain developed at Absolute Antibody for research and assay development. It contains key point mutations that abrogate binding of Fc receptors (FcγR, FcR), abolishing antibody directed cytotoxicity (ADCC) effector function.
Fc Silent™ represents a genetically engineered Fc domain developed at Absolute Antibody for research and assay development. It contains key point mutations that abrogate binding of Fc receptors (FcγR, FcR), abolishing antibody directed cytotoxicity (ADCC) effector function.
Our Fc Silent™ antibodies therefore enable researchers to remove effector function in vivo and reduce non-specific background in staining methods. In vivo, the antibodies benefit from the long plasma half-life (t1/2) associated with the Fc domain, with none of the cytolytic immune effector mechanisms associated with the wild-type Fc domain. In vitro, the antibodies can be used for flow cytometry and immunohistochemistry (IHC) applications to reduce non-specific background signal resulting from Fc receptor binding. The Fc Silent™ mutations do not affect other properties of the antibody, including their compatibility with secondary antibodies.
All Absolute Antibody antibodies and fusion proteins are available with the Fc Silent™ domain. Our reagents catalog includes species-specific Fc Silent™ domains, such as human, mouse, rabbit, rat and hamster, in stock and ready to purchase. If you don’t see your antibody of choice in the Fc Silent™ format, contact us – we’d be happy to produce it for you!
For more information about Fc receptors’ impact on effector function or Fc engineering efforts, check out our Antibody Resource, a free online reference resource for researchers.
Fc Silent™ Binding Data
Mouse IgG2a and rat IgG2b isotypes are known to bind strongly to Fc receptors irrespective of the test antibody specificity. Isotype controls are employed to allow the researcher to distinguish between specific and non-specific background signals. In Figure 1, we show how murine macrophages are labelled almost as strongly by a rat IgG2b isotype control as by an anti-F4/80. Switching to Fc Silent™ antibodies results in reliable data with background staining indistinguishable from a secondary-antibody-only control. This also shows that Fc Silent™ antibodies are compatible with standard secondary antibodies.
Figure 2 uses surface plasmon resonance (SPR) and direct competitive inhibition ELISA to show that the Fc Silent™ format does not interact with murine or human Fc receptors. This data was provided by Dr. Stephen Beers at the University of Southampton.
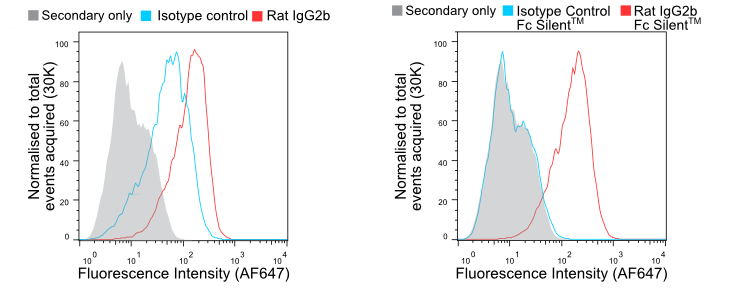
Figure 1. Flow cytometry of BMDMs stained with wild-type (A) and Fc Silent™ (B) anti-F4/80 (Ab00106-8.1 and Ab00106-8.4) and isotype control antibodies, followed by fluorescently conjugated goat anti-rat secondary antibody. Using Fc Silent™ abolishes non-specific FcγR driven staining, making data cleaner and more accurate. Click here for more information.
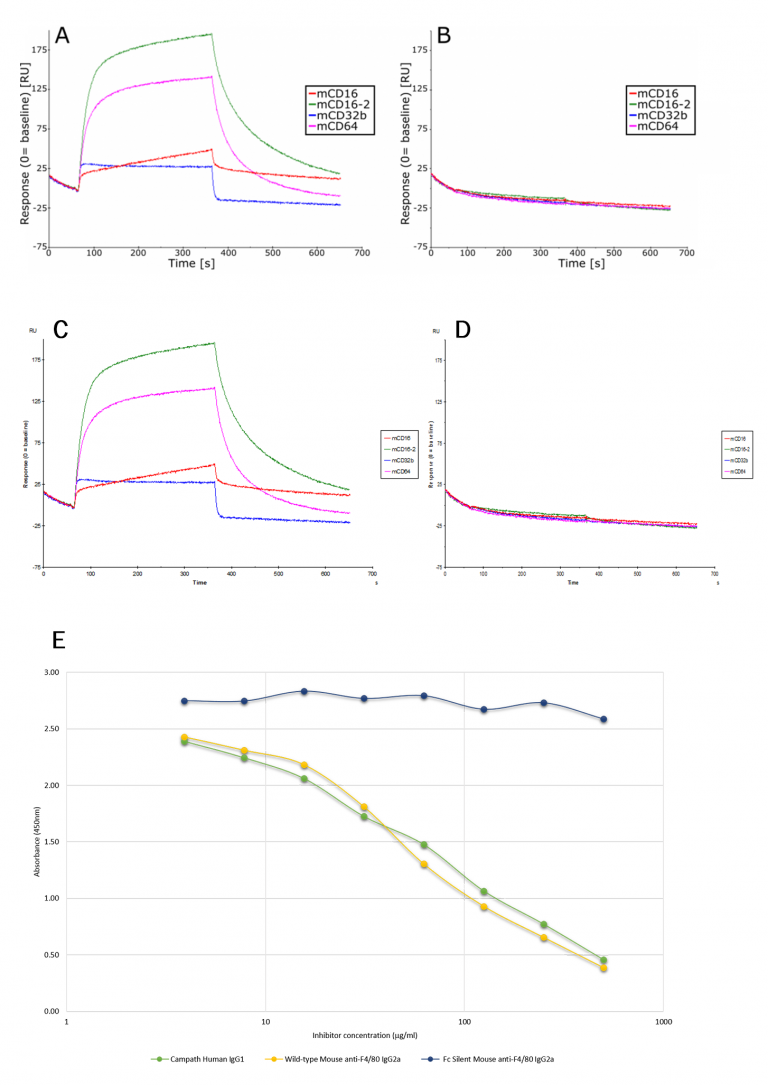
Figure 2A and B. Sensogram showing binding of mouse wild-type and Fc Silent™ anti-F4/80 IgG2a monoclonal antibodies (Ab00106-2.0 and Ab00106-2.3, respectively) to immobilized mouse low and high affinity Fcγ receptors (CD16, CD16-2, CD32, CD64). Biacore SPR binding analysis shows that the Fc Silent™ antibody has lost its capacity to interact with the mouse Fc receptors.
Figure 2C and D. Sensogram showing binding of rat wild-type and Fc Silent™ anti-F4/80 monoclonal antibodies (Ab00106-8.1 and Ab00106-8.4, respectively) to immobilized mouse low and high affinity Fcγ receptors (CD16, CD16-2, CD32, CD64). Biacore SPR binding analysis shows that the Fc Silent™ antibody has lost its capacity to interact with the mouse Fc receptors.
Figure 2E. Direct competitive inhibition ELISA. 2µg/ml/well of human recombinant FcγR1 receptor (rCD64) was absorbed onto a 96-well plate. 40µg/ml biotinylated Alemtuzumab (Campath) was premixed with un-labelled serially diluted murine wild-type or Fc Silent™ anti-F4/80 [CI:A3-1] IgG2a monoclonal antibodies (Ab00106-2.0 or Ab00106-2.3, respectively) or Alemtuzumab (Campath; positive control). The data clearly show that the Fc Silent™ antibody, Ab00106-2.3, does not bind to the human Fc receptor.
Want to read more about Fc silencing? Check out our latest blog post: Is Fc Silencing Right for Your Antibody?

 United Kingdom (UK)
United Kingdom (UK)