What is Fc silencing?
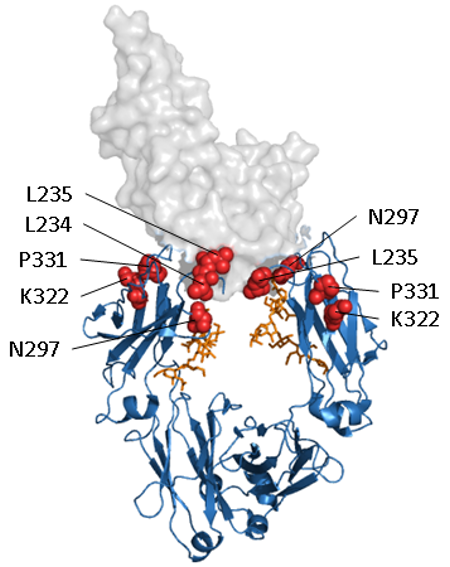
Figure 1. Abolishing Fc effector function. Structural representation of a human IgG1 Fc domain in complex with FcγRIII (PDB 1T83). The Fc domain is shown as a ribbon representation in blue, glycosylation in orange and FcγRIII as a transparent space filled model. A selection of the amino acids that have been targeted for abolishing interaction with Fc receptors are shown as red spheres.
If you have been newly introduced to antibody customization, you may be unfamiliar with the term Fc silencing. Fc silencing is the process of eliminating the binding of immunoglobulin Fc to Fc gamma receptors and complement protein C1q, thus abolishing immune effector functions (Wilkinson et al. 2021 and Schlothauer et al. 2016). This is accomplished by engineering mutations in the Fc region of the immunoglobulin. These antibodies are typically used in therapeutics to reduce FcγR activation and Fc-mediated toxicity, but they also have utility for research purposes.
How does Fc silencing work?
Fc-mediated immune effector functions are an important part of an antibody’s natural function, but in some, usually therapeutic, antibodies these interactions are not desirable and can lead to adverse effects. Early attempts to solve this problem included using IgG4 until it was realized that this subclass can undergo Fab-arm exchange, where heavy chains can be swapped between IgG4 in vivo. Numerous Fc engineering approaches have since been developed to create Fc silenced antibodies, each with different mutations on the Fc region to reduce binding to Fc gamma receptors and C1q. Figure 1 shows a selection of the amino acids (red spheres) that have been targeted for abolishing Fc receptor interactions. For example, LALA substitutions, a popular IP-free option that consists of the mutation of leucine at positions 234 and 235 into alanine, reduce binding to FcγRI, FcγRII, and FcγRIII as well as to complement component C1q. Absolute Antibody offers two custom platforms, Fc Silent™ and STR silencing, to meet your antibody silencing needs.
Fc Silent™
Fc Silent™ is a genetically engineered Fc domain developed by Absolute Antibody for use in research and assay development. Using key point mutations, binding to Fc receptors is abrogated and antibody directed cytotoxicity (ADCC) effector function is abolished. These antibodies can be used for in vivo and in vitro applications.
- In vivo: Fc Silent™ antibodies enable researchers to remove effector function while maintaining FcRn binding. This allows for a long plasma half-life without the cytolytic mechanisms associated with a wild-type Fc domain.
- In vitro: Non-specific background signaling from Fc receptor binding is reduced in flow cytometry and immunohistochemistry applications. The mutations in Fc silent antibodies do not affect other properties of the antibody, including secondary antibody compatibility.
A large portion of our recombinant antibodies and fusion proteins are available with the Fc Silent™ domain or can be customized to meet your needs. Additionally, we proudly partner with mAbsolve to offer STR Fc silencing for therapeutic antibody development.
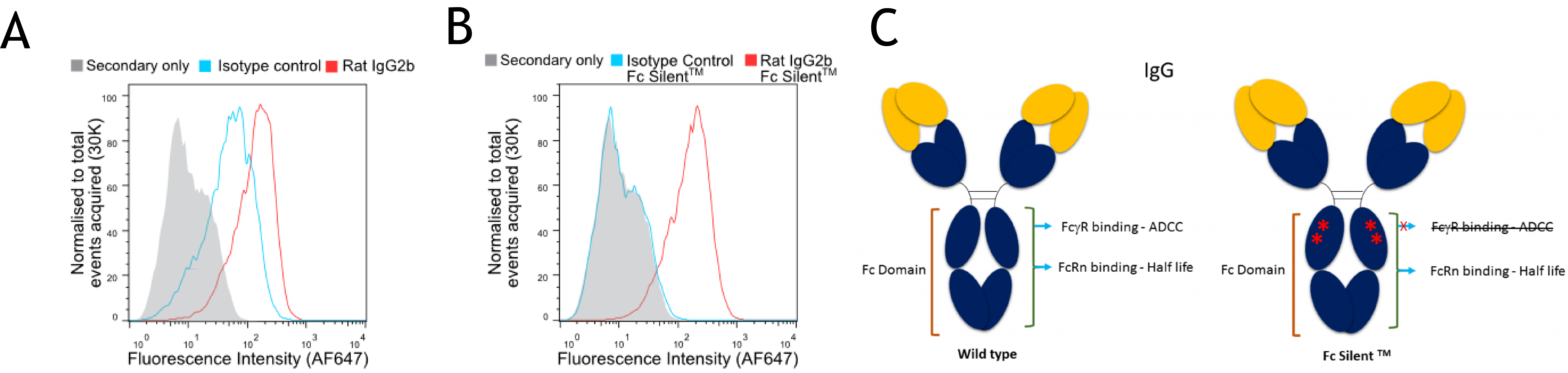
Figure 2. (A and B) Flow cytometry of BMDMs stained with wild-type (A) and Fc Silent™ (B) anti-F4/80 (Ab00106-8.1 and Ab00106-8.4) and isotype control antibodies, followed by fluorescently conjugated goat anti-rat secondary antibody. Using Fc Silent™ abolishes non-specific FcγR driven staining, making data cleaner and more accurate. (C) Comparison of wild type Fc domain and Fc Silent™ domain.
STR Silencing
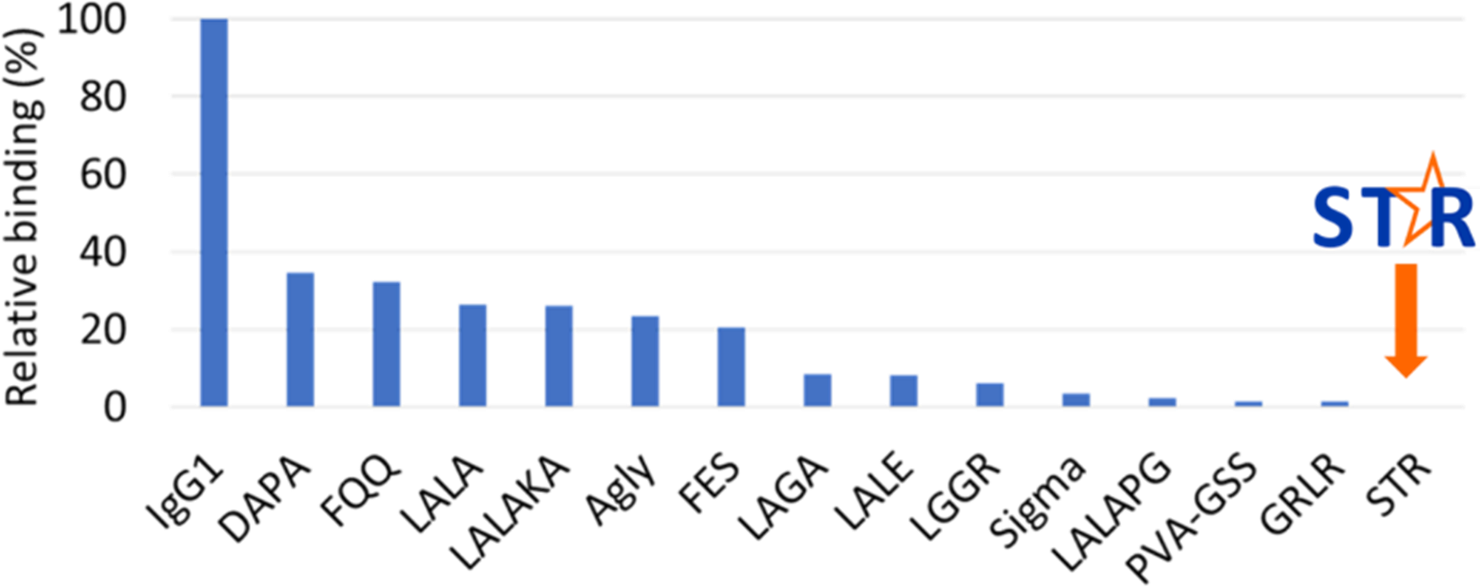
Figure 3. Binding of IgG1 variants to human FcyRI (CD64). STR is the first variant to completely eliminate binding to all Fc receptors, including FcyRI.
The vast majority of Fc silencing technologies available today do not completely abolish Fc binding and effector function (Figure 3), except for one. The STR Fc silencing platform, developed by mAbsolve and former Absolute Antibody scientists, delivers the only truly silent Fc mutations described to date. This technology has the potential to improve the safety and efficacy of therapeutic antibodies and Fc fusion proteins. Comprised of three mutations in the Fc domain, this platform can be applied to any IgG-based antibody or fusion protein and allows for the retention of all the favorable attributes of the Fc domain, while eliminating Fc receptor and C1q binding. Not only will the antibody be truly silent, but Absolute Antibody and mAbsolve scientists will provide technical support throughout the process.
For researchers looking to evaluate STR silenced antibodies, we offer several off-the-shelf options, including the research-grade biosimilars Anti-erbB-2 (Her-2/neu) [4D5-8 (trastuzumab)] and Anti-CD20 [10F381 (rituximab)] and the isotype controls Anti-Fluorescein [4-4-20 (enhanced)] and Anti-hapten 4-hydroxy-3-nitrophenyl acetyl (NP) [B1-8]. Additionally, STR mutations can be applied to our other catalog antibodies or your own custom antibodies upon request.
Conclusion
Diminishing Fc region binding, and subsequent effector function, has great utility in the therapeutics and research industries. The type of Fc silencing technology to use varies depending on your requirements and antibody application. Absolute Antibody’s team of engineering experts will consult with you to choose the best silencing option for your antibody. Learn more about our custom antibody services and contact us to discuss your next antibody project.
Latest News
Upcoming Events
Please join us at the following conferences and events. Stop by our booth, or get in touch to arrange a meeting.
See All Dates
 United Kingdom (UK)
United Kingdom (UK)