Below is a list of our latest research posters, presentations and brochures, available for download and viewing. Please contact us with any questions. If you’re looking for more general background information on antibodies and antibody engineering, be sure to check out the Antibody Resource section of our website.
Posters
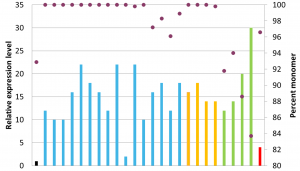 Prometheus™: a novel approach for engineering manufacturable humanized antibodies
Prometheus™: a novel approach for engineering manufacturable humanized antibodies
This poster describes our humanization approach, which was informed by analyzing manufacturability data for more than 100 therapeutic antibodies to identify preferential manufacturing properties. The poster also describes an antibody humanization case study, in which we created 25 different humanized variants with increased titers compared to the original chimeric antibody. The three winning candidates displayed no detectable aggregation, an approximately two-fold increase in activity, and a minimum 16-fold increase in titer compared to the chimeric antibody.
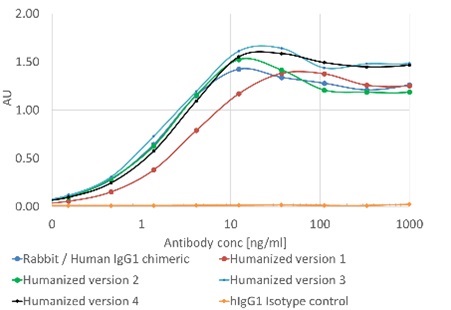 Prometheus 2.0™: engineering manufacturable humanized antibodies from diverse sources including rabbit and camelid antibodies
Prometheus 2.0™: engineering manufacturable humanized antibodies from diverse sources including rabbit and camelid antibodies
This poster details the expansion of our antibody humanization service to include humanization from non-murine origin species. We have incorporated strategies to overcome structural differences of rabbit and camelid antibodies to generate humanized antibodies with functional characteristics comparable to the original antibody, while maintaining, and often even improving, manufacturability characteristics.
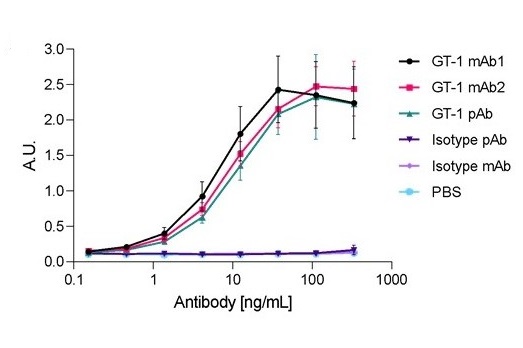 Sequencing and expression of monoclonal antibodies from a polyclonal goat antibody sample
Sequencing and expression of monoclonal antibodies from a polyclonal goat antibody sample
This poster reports the de novo sequencing of monoclonal antibodies from a polyclonal goat antibody sample by mass spectrometry, followed by recombinant expression and testing. To our knowledge, this is the first report of a successful conversion of a goat polyclonal to a monoclonal using just antibody protein as a template. We welcome a wider adoption of this approach, which with continual validation will have profound impact on the research reagents and diagnostics industry, and shows great potential for the development of therapeutics from existing polyclonal antibodies.
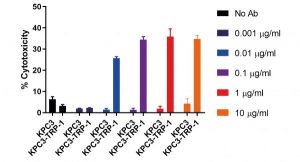
Fully murine knob-into-hole bispecific as surrogate molecule for drug development models
This poster reports the generation of a fully murine, knob-into-hole (KIH), heavy-chain heterodimerizing, bispecific antibody format. It is the first commercially available KIH bispecific antibody platform for creating fully murine surrogate molecules for syngeneic evaluation of target combinations. The poster also provides functional characterization of an anti-mCD3ε:TRP-1 bispecific antibody, capable of selectively recruiting T-cells to TRP-1+ cancer cells for increased cytotoxic effector function, as a proof of concept.
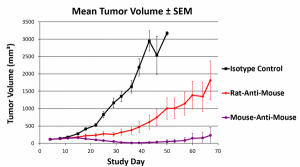 Consistent, long-term PD1 blockade using a syngeneic, engineered anti-PD1 antibody
Consistent, long-term PD1 blockade using a syngeneic, engineered anti-PD1 antibody
This poster compares the efficacy of the anti-mouse PD1 clone RMP1-14 in its original rat IgG2a format against a recombinantly engineered mouse IgG2a Fc Silent™ version at different doses over a period of 70 days in a non-immunogenic HEP1-6 liver cancer model. Syngeneic mouse IgG2a Fc Silent™ antibodies showed better dose efficacy and more homogenous treatment responses, reducing tumor size in mouse models more effectively than the traditional rat monoclonal antibody.
 Engineering novel synthetic vector components for intensive recombinant protein production in CHO cells
Engineering novel synthetic vector components for intensive recombinant protein production in CHO cells
This poster describes the generation of a novel synthetic promoter that significantly increases transient antibody expression in CHO cells. When the promoter was used in combination with other genetic vector technology, a seven-fold increase in expression was achieved compared to the existing industry-standard vector. The research was conducted in collaboration with the University of Sheffield.
An integrated workflow for recombinant antibodies in lateral flow immunoassay development
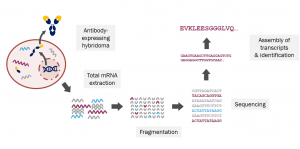
Developing a successful diagnostic test or assay requires a secure supply chain of high-quality reagents. This poster presents an integrated workflow for introducing recombinant antibodies into lateral flow immunoassay development. Recombinant antibody technology allows you to obtain highly reliable antibodies tailored to your specific assay, enabling superior performance for the entire lifespan of your diagnostic.
Absolute Antibody has engineered and produced more than 180 different antibody formats for our customers, as illustrated by our Periodic Table of Antibodies. The antibody formats are categorized in the table by number of specificities, starting with single target antibodies and moving into bispecific and trispecific antibodies. The antibodies are further categorized as Fc containing antibodies, antibody fragments and Fc fusion proteins. Learn what each format is by downloading our Periodic Table of Antibodies poster with the full legend.
Comparing potential bispecific formats comprising of trastuzumab and a humanized OKT3 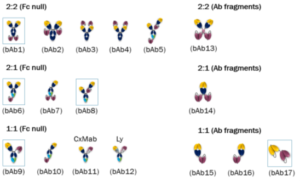
Not every antibody can be combined to produce well-behaved multispecifics. The valency and geometry of each design can determine the production, target engagement and ultimately the requisite biological functions. In this case study, we selected two established antibody therapeutics, trastuzumab and a humanized OKT3, to produce 17 different bispecific formats to compare the feasibility of each format.
White Papers and Application Notes
 An Introduction to Recombinant Antibody Technology for Diagnostics Applications
An Introduction to Recombinant Antibody Technology for Diagnostics Applications
This white paper provides an overview of recombinant antibodies and their utilization in the development of diagnostic tests and immunoassays. It discusses different types of antibodies, introduces recombinant antibody technology, and describes the benefits recombinant antibodies can offer to diagnostic developers.
 A Question of Isotype: How Switching Antibody Isotypes and Fc Regions Promotes Discovery
A Question of Isotype: How Switching Antibody Isotypes and Fc Regions Promotes Discovery
This white paper discusses how antibody isotype and subtype switching and Fc region alteration can make antibodies better suited for cancer research in live mouse models. It explores optimal antibody formats for various immunotherapy targets, relative receptor binding affinities of different subtypes, and how tailored effector function affects antibody therapies.
9 Questions to Consider Before Your Next Antibody Engineering Project
This white paper provides an overview of 9 topics to consider before beginning a new antibody engineering project. It discusses various antibody formatting options, half-life and Fc effector functions, manufacturability, and recombinant expression platforms.
Guide to Recombinant Antibody Engineering
This white paper provides an overview of recombinant antibody engineering. It covers key antibody background, then describes the different design parameters to consider when engineering an antibody. We then outline different antibody engineering options, including humanization, fragments, multispecifics, and Fc fusion proteins. Finally, we look at recent industry developments and the future of antibody engineering.
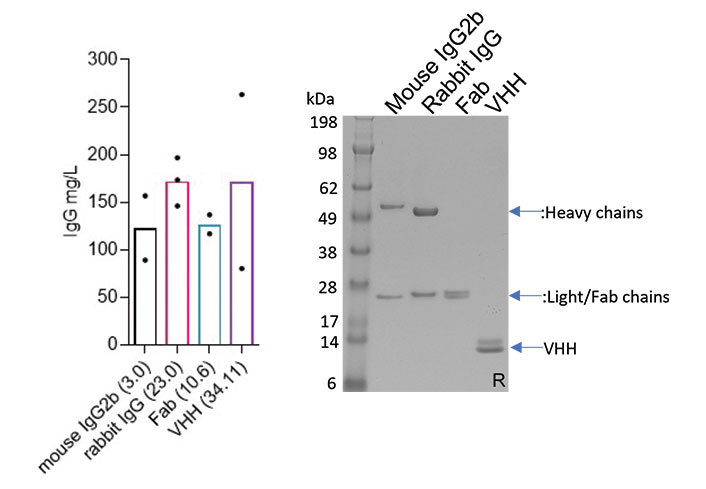
Development and characterization of a transient CHO expression platform for recombinant antibody production
This application note reviews the quality and batch-to-batch reproducibility of the recombinant antibodies manufactured with Absolute Antibody’s CHXpress™ transient CHO platform. Data demonstrate that the platform produces high quality, fully glycosylated human IgG antibodies with high consistency between batches, as well as antibodies in engineered formats with reliably robust production yields and expected binding activity.
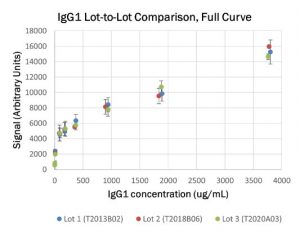
Transient antibody expression platform drives batch-to-batch reproducibility for COVID-19 diagnostic assay
This application note looks at the batch-to-batch reproducibility of our anti-coronavirus spike glycoprotein antibody CR3022. We sought to determine if there were any significant differences in signals between three lots and three isotypes of the antibody when run on an external COVID-19 antibody test. Batch-to-batch reproducibility and stability of the recombinant protein were confirmed.
Presentations
 Approaching bispecific antibody engineering
Approaching bispecific antibody engineering
Presenter: Michael Fiebig, PhD, CSO, Absolute Antibody
Designing bispecific antibodies is a complex process, but some common challenges of generating bispecific antibodies have been tackled by protein engineers with creative approaches. This presentation covers suggested initial go-to formats for bispecific antibodies, as well as the utility of mouse bispecifics in basic research and drug discovery.

Why go recombinant? Take your experiments further with high reproducibility and antibody engineering
Presenter: Michael Fiebig, PhD, VP Product Portfolio & Innovation, Absolute Antibody
Recombinant antibodies offer significant benefits compared to traditional hybridoma-produced monoclonal antibodies, including ensuring batch-to-batch reproducibility and enabling antibody engineering. Antibody engineering allows researchers to improve experimental design and develop complex controls with absolute precision.
 Taking the training wheels off transient expression: rapid antibody production for pandemics
Taking the training wheels off transient expression: rapid antibody production for pandemics
Presenter: Ian Wilkinson, PhD, Absolute Antibody CSO
In a pandemic, three critical things need to be developed quickly: diagnostics, therapeutics and vaccines. Transient expression of antibodies and other proteins offers an important tool for rapid scale-up and production. It is a well-established technique that, if applied correctly, could make future pandemic responses quicker.
 Scaling up and scaling out: pushing the boundaries of transient protein production
Scaling up and scaling out: pushing the boundaries of transient protein production
Presenter: Ian Wilkinson, PhD, Absolute Antibody CSO
While transient yields have improved drastically in the last decade, scalable systems are time-consuming and costly to implement. Absolute Antibody has developed systems which scale up and scale out protein expression and purification, enabling the rapid and cost-effective production of milligram-to-gram quantities of large panels of proteins.
Comparing potential bispecific formats of trastuzumab and a humanized OKT3
Presenter: Donmienne Leung, PhD, Head of Protein Engineering, Absolute Antibody
Not every antibody can be combined to produce well-behaved multispecifics. The valency and geometry of each design can determine the production, target engagement and ultimately the requisite biological functions. In this case study, we selected two established antibody therapeutics, trastuzumab and a humanized OKT3, to produce 17 different bispecific formats to compare the feasibility of each format.
Applying Recombinant Antibody Engineering to Further Immunoassay Development
Presenter: Alex Scott, MChem, Business Development Manager, Absolute Biotech
Recombinant antibody technology allows diagnostic and immunoassay developers to obtain highly reliable and tailored antibodies, enabling superior performance for the entire lifespan of a diagnostic product. This presentation focuses on how antibody engineering allows assay developers to custom design antibodies to improve their performance in rapid diagnostic tests.
Product and Service Brochures
- Recombinant Antibody Services
- Recombinant Antibody Catalog
- Multi-Hybridoma Conversion Service
- Recombinant Antibodies for Diagnostic Developers
- VivopureX™ Recombinant Antibodies for In Vivo Research
- Recombinant Coronavirus Antibodies
- Murine Bispecific Antibody Reagents
- Fully Silent Fc-Engineered Antibodies
- CHXpress™ Antibody Expression

 United Kingdom (UK)
United Kingdom (UK)