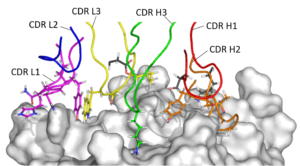
Humanization is a critical step in the development pathway of all therapeutic antibodies derived from non-human sources, such as hybridomas. Absolute Antibody’s Prometheus™ antibody humanization service delivers a panel of humanized variants from your clone, guaranteeing to produce a variant with comparable activity to the parent antibody. Our service focuses on enhancing antibody manufacturability, leading to increased expression, lower aggregation, and long-term stability and solubility.
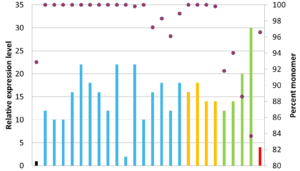
Humanized antibody variant expression yields relative to the original chimeric antibody, shown in black. Download our research poster describing our antibody humanization approach to learn more.
Our Humanization Approach
Antibody humanization involves the transfer, or grafting, of critical non-human amino acids onto a human antibody framework. This primarily includes the grafting of amino acids in the complementarity-determining regions (CDRs), but also other framework amino acids critical for the VH:VL interface and CDR orientation. Humanization is a balance between introducing as much human content as possible to reduce the risk of immunogenicity, while retaining enough non-human content to maintain the original binding activity of the parent antibody.
Another key consideration is the developability of the antibody, which is a combination of immunogenicity and manufacturability. Manufacturability encompasses a range of properties such as expression titer, aggregation, stability and solubility, and Absolute Antibody’s Prometheus™ humanization service was developed specifically to deliver antibodies with increased manufacturability.
Download our research poster to learn more about the development of our humanization approach, which was informed by analyzing manufacturability data for more than 145 therapeutic antibodies to identify preferential manufacturing properties. The poster also describes an antibody humanization case study, in which we created 25 different humanized variants with increased titers compared to the original chimeric antibody. The three winning candidates displayed no detectable aggregation, an approximately two-fold increase in activity, and a minimum 16-fold increase in titer compared to the chimeric antibody.

State University of New York researcher, describing our services. Read his full antibody humanization case study here.
Advantages of Our Prometheus™ Humanization Service
The Absolute Antibody team has deep antibody humanization expertise, with some of our founders and scientific advisors even humanizing Campath-1H, the first humanized antibody to be used in the clinic. We offer a royalty-free humanization service with a guarantee of success – if we fail to deliver a humanized antibody with comparable activity to the parent antibody, you will not be charged for the service.
Our service is uniquely focused on delivering humanized antibodies with strong manufacturability, enabling the optimized large-scale production required for clinical use. We guarantee to produce a panel of humanized variants, and we have completed more than 145 successful humanization projects to date, for clients ranging from small academic laboratories to leading pharmaceutical companies.
In addition, while our approach was initially focused on humanizing mouse or rat antibodies, we have since expanded our service to offer humanization from non-murine origin species, including rabbit antibodies and single-domain camelid nanobodies. Download our second humanization poster to learn more about humanizing antibodies from non-traditional sources.
To learn more about our process and the benefits to our approach, check out this customer case study, which describes how we helped a State University of New York researcher humanize an antibody with the potential to treat glioblastoma.
Our Humanization Deliverables
If required, antibody sequences can be determined with our hybridoma sequencing or de novo protein sequencing services. Alternatively, customers can come directly to us with a sequence.
An in silico model of the parental variable domains is generated to enable structure-guided humanization. Sequences are aligned to a panel of human germline sequences selected for preferential manufacturability properties, and the non-human amino acids are grafted onto the human sequence using our proprietary Prometheus™ humanization algorithm.
We offer four levels of service, ranging from in silico humanization only to a full expression, purification and analytics package, as shown in the table below. Contact us to discuss your project today.
Interested in more information about antibody humanization? Check out our latest blog post: Antibody Humanization to Combat Antimicrobial Resistant Bacteria.

 United Kingdom (UK)
United Kingdom (UK)