The preclinical path for cancer therapy is paved by in vitro and in vivo studies, and lab-grown cells are crucial to the experimental process for both. Cultured cells can be used as tumor models to explore the mechanisms of action that surround tumor progression and as subjects on which to test potential therapeutic drugs. Implanting cell lines into live model organisms, in particular mice, is an established method to simulate tumors in a reliable, well-characterized way to explore the holistic effects of tumors and anti-tumor therapies alike.
The use of appropriate cell lines and antibodies together is critical for preclinical research, as it ensures the validity and reliability of results, provides a better understanding of the target biology, optimizes experimental conditions, and improves the translation of preclinical results to clinical trials. Absolute Antibody provides thousands of recombinant antibodies, with our VivopureX™ collection engineered specifically for optimized in vivo performance, and related cell lines are available through our sister company Kerafast. This article outlines our top recommendations for cell line and antibody combinations to power your research.
Cell Lines in Cancer Research:
The right cell lines can open experimental avenues to gain the understanding needed to move research forward. Some specific examples of how cell lines are used in cancer treatment research include:
- Immunotherapy: Cultured cells can be used to model cancer to evaluate the efficacy of immune checkpoint inhibitors, such as anti-PD-1 or anti-CTLA-4 antibodies. These inhibitors can block the interaction between cancer cells and T cells, thereby enhancing the immune response against the tumor.
- Targeted therapy: Cultured cells can also be used to evaluate the efficacy of therapies that specifically target molecules that are overexpressed or mutated in cancer cells.
- Combination therapy: Cultured cells are often used to test the efficacy of combination therapies, where multiple treatments are used in combination to enhance their effectiveness.
- Drug discovery: Cultured cells can also be used as a model to discover new drugs and to identify potential targets for cancer therapy.
The following are examples of cell lines commonly used in tandem with antibodies to further our understanding of cancer immunotherapy.
MC-38 Cells
MC-38 cells were originally developed and extracted from a colon tumor in a C57BL/6 mouse exposed to the carcinogen DMH by Corbett et al. in 1975 to provide researchers with effective syngeneic mouse adenocarcinoma models. Since then, this cell line has empowered cancer research in more than 2,500 studies. MC-38 cells are highly characterized as a predictive model (Corbett et al., 1975; Taylor et al., 2019), exhibit tumor heterogeneity similar to human tumors (Taylor et al., 2019), and are immunogenic (Yadav et al., 2014).
MC-38 cells are commonly used as a preclinical model in cancer treatment research to evaluate the efficacy and safety of various anti-cancer therapies, including chemotherapy, radiation therapy, immunotherapy, and targeted therapies. MC-38 cells and related antibodies have furthered our understanding of cancer and anti-cancer therapies in a variety of ways.
For example, MC-38 cells can express PD-L1, a ligand for PD-1 (programmed cell death protein 1) which attenuates immune response. Many antibody therapies aim to block the programmed cell death pathway by targeting PD-1 or its associated ligand in order to prevent the reduction of anti-tumor activity. In the case of this 2021 study, MC-38 cells were used as an in vivo tumor model to evaluate a combination therapy of CCR8-targeting nanobodies applied in conjunction with anti-PD-1. The combination therapy was found to be effective at depleting tumor-infiltrating Tregs. A murine Fc-silenced version of anti-mouse PD-1 monoclonal antibody clone RMP1-14 (Ab00813-1.32) was used due to its mechanistic similarities to clinical anti-PD-1 therapies.
Additionally, this 2020 study used MC-38 cells as tumor models in live mice to image and determine the optimized dose of an anti-CD47/PD-L1 bispecific antibody therapeutic (see our bispecific antibody page for your own bispecific options, or take a look at the anti-PD-L1 and anti-CD47 antibodies optimized for experiments in live mice). The MC-38 cell line can help the exploration of multispecific antibody therapies in live mice models for effective preclinical research.
MC-38 cells can also be generated to express additional genes, as in the case of this 2023 study that took MC-38 cells from Kerafast and transfected them with a TRP1-coding plasmid before infecting mice with the MC-38(.TRP1) tumors. A murine knob-in-hole bispecific antibody that targets CD3 and TRP1 (bAb0136) with a silenced Fc region developed by Absolute Antibody was administered in conjunction with reovirus to explore the optimum tumor environment for this immunotherapy. The cell line and bispecific antibody provided these researchers the right tools for their in vivo study to further our understanding of potential cancer immunotherapies.
KPCY Cells
Pancreatic ductal adenocarcinoma (PDA) is a type of exocrine pancreatic cancer that is the seventh leading cause of cancer deaths worldwide (Ushio et al., 2021). PDA develops from cells lining small tubes in the pancreas called ducts. These carry digestive juices, which contain enzymes, into the main pancreatic duct and then on into the duodenum. PDA can grow anywhere in the pancreas, though it is most often found in the head of the pancreas. To study this disease, researchers need the right cell lines for the job. KPCY cells are cells taken from the pancreatic ductal adenocarcinoma of a mouse positive for the Kras (K), Trp53 (P), Cre (C), and yellow fluorescent protein (Y) genes. Mutated Kras and p53 (coded for by Trp53) are implicated in the carcinogenesis of pancreatic cancer and are recapitulated faithfully by KPCY cells (Hingorani et al., 2005; Ushio et al., 2021). This makes them valuable tools for exploring therapeutic options for this form of cancer.
Like MC-38 cells, KPCY cells can be used in live mouse models as a cancer model to investigate the effects of immunotherapies on tumorigenesis. In this 2011 study, anti-CD40 monoclonal; antibodies were used to observe the effect of their application on KPC mouse tumors in vivo. The data show that CD40-directed antibodies had an agonistic effect on tumor-infiltrating macrophages, mediating tumor regression. This mechanism of action is a departure from more common immune checkpoint inhibitors, as agonistic CD40-directed antibodies actively promote T cell responses against tumors rather than block negative immune responses (Vonderheide & Glennie, 2013). We offer the same anti-CD40 antibody clone recombinantly in a variety of murine isotypes and effector function for your research.
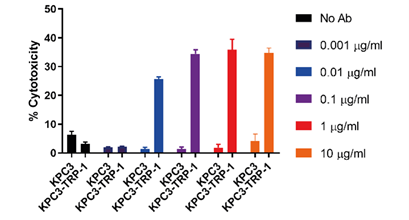
Figure 2. Our anti-CD3ε:TRP-1 bispecific engineered antibodies are effective anti-tumor treatments in KPC-TRP+ tumor cells in live murine models.
In terms of immune checkpoint inhibitors, KPCY cells have helped our understanding of how this class of antibodies can enhance other therapies. PDA has historically been a difficult cancer to treat in human patients and responds poorly to immunotherapy alone. This 2013 study found that when the chemokine CXCL12 is applied in tandem with in mice, the effects are synergistic and the tumor cells are greatly diminished. This 2020 study also looked into combination therapy efficacy and evaluated how estrogen signaling affects the progression of PDA, finding that activating the G protein-coupled estrogen receptor (GPER) with a synthetic molecule reduced tumor progression in mice. Furthermore, pairing pharmacological activation of the GPER with anti-PD-1 monoclonal antibody application greatly increased the efficacy of both therapies.
KPCY cells are yet another tool that, when paired with antibody technology, open up new experimental opportunities for research. Our study on knob-in-hole bispecific anti-CD3ε:TRP-1 antibodies that we engineered were found to effectively destroy KPCY-TRP+ tumor cells implanted in live mice (Fig. 2). KPCY cells enabled our engineers to determine the effectiveness of our bispecific platform in vivo, opening up new opportunities for research into potential antibody therapies.
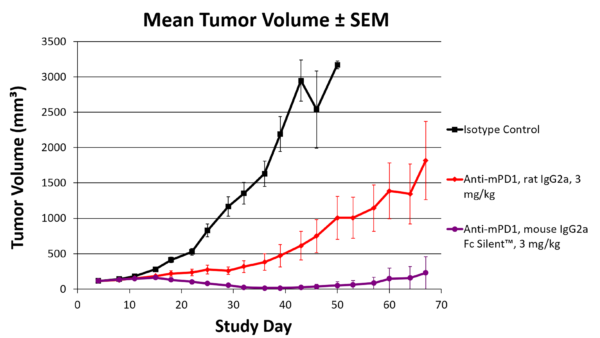
Figure 3. Our syngeneic engineered anti-PD-1 antibody performs better at reducing tumor volume than the original rat-derived anti-PD-1 antibody in live mice.
Additionally, our VivopureX™ antibodies are recombinantly produced and engineered to work as effectively as possible in live mouse models, enabling better cancer research and cleaner data (Fig. 3). Pair KPCY cells with anti-PD-1 and anti-PD-L1 antibodies in the most clinically relevant formats for your cancer research.
OSCC Cells
Oral squamous cell carcinoma (OSCC) is the sixth most common cancer worldwide and encompasses most head and neck cancers. A distinct trait of OSCC is that this cancer is most often developed in response to carcinogen exposure rather than human papillomavirus (Sathiyasekar et al., 2016). Moreover, while surgery, chemotherapy, and radiation therapy have improved, OSCC prognoses have not improved in the past two decades (Chiu et al., 2022). This makes the need for better therapies more pressing.
OSCC cell lines are helping advance this field of research, with cell lines available that have been characterized by the aggressiveness of their growth rates. MOC2 cells, offered by Kerafast from the lab of Dr. Ravindra Uppaluri at Washington University in St. Louis, grow aggressively and can form tumors in live murine models with injections of fewer than 10,000 cells. MOC1 and MOC22 cell lines from the same source are characterized by indolent growth patterns.
A study published in 2020 implanted MOC1 and MOC2 cells from Kerafast into mice to model OSCC tumors, then applied engineered natural killer cells that express the PD-L1 chimeric antigen receptor, finding that these cells reduced tumor size, dependent on CD8 and PD-L1. Important to note is that the researchers used engineered antibodies to determine that the effectiveness of this therapy depended on CD8 and PD-L1. We provide the same clones in clinically significant, recombinant formats built for in vivo use to further explore OSCC treatment options.
In addition, a unique application of MOC cells and antibodies for oral squamous adenocarcinoma treatment is described in this 2017 article. Anti-CD44 antibodies were conjugated to a photoabsorber to guide near-infrared photoimmunotherapy in MOC1 and MOC2 cells which express CD44. This resulted in significantly reduced tumor growth. As these studies show, MOC cells can be paired with antibodies for a wide range of experimental options. View our available engineered formats for CD8, PD-L1, and CD44 antibodies here.
Accessing Your Preferred Cell Lines and Antibodies
The right cells with the right antibody reagents can empower your research, as the studies above illustrate. However, not all cell lines are equally suited to furthering your research goals. Ideally you want to select well-characterized cell lines with years of demonstrated success in peer-reviewed publications. However, many such cell lines come from academic laboratories and can be difficult to source. Kerafast, our sister company under Absolute Biotech, facilitates access to unique lab-made reagents such as cell lines, ensuring researchers worldwide have easy access to valuable research tools.
Add even more reliability to your experiments with recombinant engineered antibodies from Absolute Antibody. With recombinant technology, each antibody’s sequence is absolutely defined, immortalized, and produced to your exact specifications in off-the-shelf and custom formats. For studies in live mouse models, our VivopureX™ line offers the most up-to-date formats for cutting-edge research, optimized for in vivo experimentation so you can worry about fewer variables and more results.
The following are a few of our recommendations for cell lines and corresponding antibodies to move your preclinical research forward. The MC-38, KPCY, and OSCC cell lines can all be accessed through Kerafast. If you have any questions or would like to request an order quote, please get in touch.
| Antibody | Cell Lines | Antibody Formats | Top Application |
|---|---|---|---|
| Anti-OX40/CD134 [OX86] | MC-38, KPCY | Mouse IgG1 | Agonism |
| Anti-OX40/CD134 [OX86] | MC-38, KPCY | Mouse IgG2a | Agonism |
| Anti-PD-1 [RMP1-14] | MC-38, OSCC, KPCY | Mouse IgG2a Fc Silent™ | Blocking |
| Anti-CTLA-4 [9D9] | MC-38, OSCC, KPCY | Mouse IgG2a | Blocking, Treg depletion |
| Anti-CTLA-4 [9D9] | MC-38, OSCC, KPCY | Mouse IgG2a Fc Silent™ | Blocking, peripheral Treg expansion |
| Anti-GITR [DTA-1] | MC-38 | Mouse IgG2a | Agonism |
| Anti-CD25 [PC-61.5.3] | MC-38 | Mouse IgG2a | Depletion |
| Anti-TIGIT [1B4] | MC-38, KPCY | Mouse IgG1 | Blocking |
| Anti-TIGIT [1B4] | MC-38, KPCY | Mouse IgG2a | Blocking, reverse activation of myeloid cells |
| Anti-Lag3 [C9B7W] | MC-38 | Mouse IgG2a Fc Silent™ | Blocking |
| Anti-IL-10R [1B1.3a] | OSCC | Mouse IgG2a Fc Silent™ | Blocking |
| Anti-Tim-3 [2C12] | MC-38, OSCC | Mouse IgG2a Fc Silent™ | Blocking |
| Anti-CD115/M-CSFR [AFS98] | MC-38, KPCY | Mouse IgG2a | Depletion |
| Anti-PD-L1 [10F.9G2] | MC-38, OSCC, KPCY | Mouse IgG2b | Blocking |
| Anti-PD-L1 [10F.9G2] | MC-38, OSCC, KPCY | Mouse IgG2b Fc Silent™ | Blocking |
| Anti-VISTA [13F3] | MC-38 | Mouse IgG2b Fc Silent™ | Blocking |
| Anti-CD20 [18B12] | MC-38 | Mouse IgG2a | Depletion |
| Anti-CD4 epitope A [YTS 177.9] | MC-38, OSCC, KPCY | Mouse IgG2a | Depletion |
| Anti-CD4 epitope A [YTS 191.1] | MC-38, OSCC, KPCY | Mouse IgG2a | Depletion |
| Anti-CD8 alpha/Lyt-2 [YTS 169.4] | OSCC | Mouse IgG2a | Depletion |
| Anti-Ly6G [1A8] | MC-38, KPCY | Mouse IgG2a | Depletion |
| Anti-CD200R [OX131] | OSCC, KPCY | Mouse IgG1 Fc Silent™ | Blocking |
| Anti-Ly6G/Ly6C [RB6-8C5] | MC-38, KPCY | Mouse IgG2a | Depletion |
| Anti-CD47 [mIAP301] | MC-38 | Mouse IgG2b Fc Silent™ | Blocking |
Latest News
Upcoming Events
Please join us at the following conferences and events. Stop by our booth, or get in touch to arrange a meeting.
See All Dates
 United Kingdom (UK)
United Kingdom (UK)